Perspectives
Arthropod-borne viruses, i.e., arboviruses, are viruses that are maintained in nature through biological transmission between susceptible vertebrate hosts by blood feeding arthropods (mosquitoes, psychodids, ceratopogonids, and ticks). Vertebrate infection occurs when the infected arthropod takes a blood meal. The term 'arbovirus' has no taxonomic significance. Arboviruses that cause human encephalitis are members of three virus families: the Togaviridae (genus Alphavirus), Flaviviridae, and Bunyaviridae.All arboviral encephalitides are zoonotic, being maintained in complex life cycles involving a nonhuman primary vertebrate host and a primary arthropod vector. These cycles usually remain undetected until humans encroach on a natural focus, or the virus escapes this focus via a secondary vector or vertebrate host as the result of some ecologic change. Humans and domestic animals can develop clinical illness but usually are "dead-end" hosts because they do not produce significant viremia, and do not contribute to the transmission cycle. Many arboviruses that cause encephalitis have a variety of different vertebrate hosts and some are transmitted by more than one vector. Maintenance of the viruses in nature may be facilitated by vertical transmission (e.g., the virus is transmitted from the female through the eggs to the offspring).
|
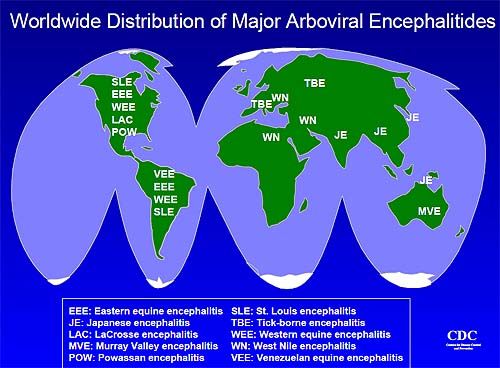
Arboviral encephalitides have a global distribution (see map below), but there are four main virus agents of encephalitis in the United
States: eastern
equine encephalitis (EEE), western equine encephalitis (WEE), St. Louis encephalitis (SLE) and La Crosse (LAC) encephalitis, all of which
are transmitted by mosquitoes. Another virus, Powassan, is a minor cause of encephalitis in the northern United States, and is transmitted
by ticks. A new Powassan-like virus has recently been isolated from deer ticks. Its relatedness to Powassan virus and its ability to cause
disease has not been well documented. Most cases of arboviral encephalitis occur from June through September, when arthropods are most
active. In milder (i.e., warmer) parts of the country, where arthropods are active late into the year, cases can occur into the winter
months.
The majority of human infections are asymptomatic or may result in a nonspecific flu-like syndrome. Onset may be insidious or sudden with fever, headache, myalgias, malaise and occasionally prostration. Infection may, however, lead to encephalitis, with a fatal outcome or permanent neurologic sequelae. Fortunately, only a small proportion of infected persons progress to frank encephalitis. Experimental studies have shown that invasion of the central nervous system (CNS), generally follows initial virus replication in various peripheral sites and a period of viremia. Viral transfer from the blood to the CNS through the olfactory tract has been suggested. Because the arboviral encephalitides are viral diseases, antibiotics are not effective for treatment and no effective antiviral drugs have yet been discovered. Treatment is supportive, attempting to deal with problems such as swelling of the brain, loss of the automatic breathing activity of the brain and other treatable complications like bacterial pneumonia.
|
There are no commercially available human vaccines for these U.S. diseases. There is a Japanese encephalitis vaccine available in the U.S. A tick-borne encephalitis vaccine is available in Europe. An equine vaccine is available for EEE, WEE and Venezuelan equine encephalitis (VEE). Arboviral encephalitis can be prevented in two major ways: personal protective measures and public health measures to reduce the population of infected mosquitoes. Personal measures include reducing time outdoors particularly in early evening hours, wearing long pants and long sleeved shirts and applying mosquito repellent to exposed skin areas. Public health measures often require spraying of insecticides to kill juvenile (larvae) and adult mosquitoes.
Selection of mosquito control methods depends on what needs to be achieved; but, in most emergency situations, the preferred method to achieve maximum results over a wide area is aerial spraying. In many states aerial spraying may be available in certain locations as a means to control nuisance mosquitoes. Such resources can be redirected to areas of virus activity. When aerial spraying is not routinely used, such services are usually contracted for a given time period.
Financing of aerial spraying costs during large outbreaks is usually provided by state emergency contingency funds. Federal funding of emergency spraying is rare and almost always requires a federal disaster declaration. Such disaster declarations usually occur when the vector-borne disease has the potential to infect large numbers of people, when a large population is at risk and when the area requiring treatment is extensive. Special large planes maintained by the United States Air Force can be called upon to deliver the insecticide(s) chosen for such emergencies. Federal disaster declarations have relied heavily on risk assessment by the CDC.
Laboratory diagnosis of human arboviral encephalitis has changed greatly over the last few years. In the past, identification of antibody relied on four tests: hemagglutination-inhibition, complement fixation, plaque reduction neutralization test, and the indirect fluorescent antibody (IFA) test. Positive identification using these immunoglobulin M (IgM) - and IgG-based assays requires a four-fold increase in titer between acute and convalescent serum samples. With the advent of solid-phase antibody-binding assays, such as enzyme-linked immunosorbent assay (ELISA), the diagnostic algorithm for identification of viral activity has changed. Rapid serologic assays such as IgM-capture ELISA (MAC-ELISA) and IgG ELISA may now be employed soon after infection. Early in infection, IgM antibody is more specific, while later in infection, IgG antibody is more reactive. Inclusion of monoclonal antibodies (MAbs) with defined virus specificities in these solid phase assays has allowed for a level of standardization that was not previously possible.
Virus isolation and identification have also been useful in defining viral agents in serum, cerebrospinal fluid and mosquito vectors. While virus isolation still depends upon growth of an unknown virus in cell culture or neonatal mice, virus identification has also been greatly facilitated by the availability of virus-specific MAbs for use in IFA assays. Similarly, MAbs with avidities sufficiently high to allow for specific binding to virus antigens in a complex protein mixture (e.g., mosquito pool suspensions) have enhanced our ability to rapidly identify virus agents in situ. While polymerase chain reaction (PCR) has been developed to identify a number of viral agents, such tests have not yet been validated for routine rapid identification in the clinical setting.
Mosquito-borne encephalitis offers a rare opportunity in public health to detect the risk of a disease before it occurs and to intervene to reduce that risk substantially. The surveillance required to detect risk is being increasingly refined by the potential utilization of these new technologies which allows for rapid identification of dangerous viruses in mosquito populations. These rapid diagnostic techniques used in threat recognition can shorten public health response time and reduce the geographic spread of infected vectors and thereby the cost of containing them. The Arbovirus Diseases Branch of NCID's Division of Vector-Borne Infectious Diseases has responsibility for CDC's programs in surveillance, diagnosis, research and control of arboviral encephalitides.
La Crosse Encephalitis
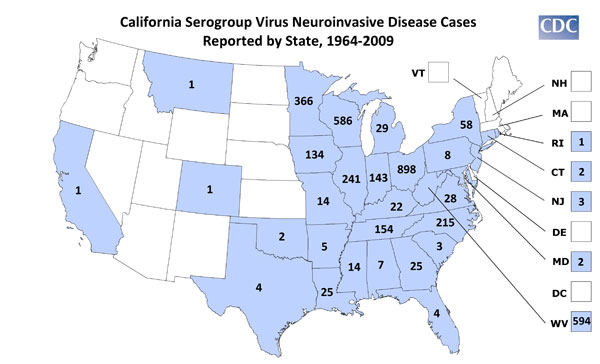
La Crosse (LAC) encephalitis was discovered in La Crosse, Wisconsin in 1963. Since then, the virus has been identified in several Midwestern and Mid-Atlantic states. During an average year, about 75 cases of LAC encephalitis are reported to the CDC. Most cases of LAC encephalitis occur in children under 16 years of age. LAC virus is a Bunyavirus and is a zoonotic pathogen cycled between the daytime-biting treehole mosquito, Aedes triseriatus, and vertebrate amplifier hosts (chipmunks, tree squirrels) in deciduous forest habitats. The virus is maintained over the winter by transovarial transmission in mosquito eggs. If the female mosquito is infected, she may lay eggs that carry the virus, and the adults coming from those eggs may be able to transmit the virus to chipmunks and to humans.
 The treehole mosquito (Aedes triseriatus) transmits the virus that causes La Crosse encephalitis. |
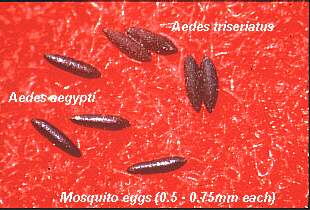 These are eggs of Aedes mosquitoes. They are laid singly on damp surfaces near water. The shiny eggs belong to Aedes aegypti, the mosquito that transmits yellow fever and dengue fever. The rough or matte eggs belong to Aedes triseriatus, the eastern treehole mosquito that transmits La Crosse encephalitis. |
Historically, most cases of LAC encephalitis occur in the upper Midwestern states (Minnesota, Wisconsin, Iowa, Illinois, Indiana, and Ohio). Recently, more cases are being reported from states in the mid-Atlantic (West Virginia, Virginia and North Carolina) and southeastern (Alabama and Mississippi) regions of the country. It has long been suspected that LAC encephalitis has a broader distribution and a higher incidence in the eastern United States, but is under-reported because the etiologic agent is often not specifically identified.
LAC encephalitis initially presents as a nonspecific summertime illness with fever, headache, nausea, vomiting and lethargy. Severe disease occurs most commonly in children under the age of 16 and is characterized by seizures, coma, paralysis, and a variety of neurological sequelae after recovery. Death from LAC encephalitis occurs in less than 1% of clinical cases. In many clinical settings, pediatric cases presenting with CNS involvement are routinely screened for herpes or enteroviral etiologies. Since there is no specific treatment for LAC encephalitis, physicians often do not request the tests required to specifically identify LAC virus, and the cases are reported as aseptic meningitis or viral encephalitis of unknown etiology.
Also found in the United States, Jamestown Canyon and Cache Valley viruses are related to LAC, but rarely cause encephalitis.
Eastern Equine Encephalitis
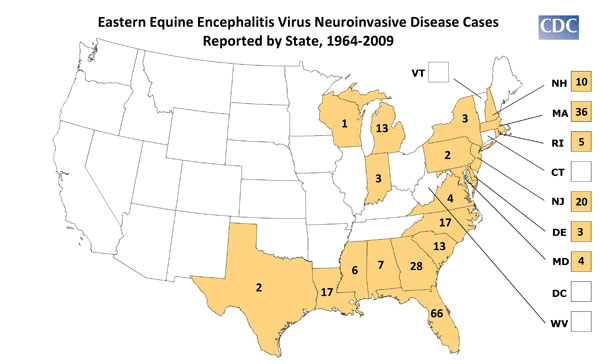
Eastern equine encephalitis (EEE) is also caused by a virus transmitted to humans and equines by the bite of an infected mosquito. EEE virus is an alphavirus that was first identified in the 1930's and currently occurs in focal locations along the eastern seaboard, the Gulf Coast and some inland Midwestern locations of the United States. While small outbreaks of human disease have occurred in the United States, equine epizootics can be a common occurrence during the summer and fall.
It takes from 4-10 days after the bite of an infected mosquito for an individual to develop symptoms of EEE. These symptoms begin with a sudden onset of fever, general muscle pains, and a headache of increasing severity. Many individuals will progress to more severe symptoms such as seizures and coma. Approximately one-third of all people with clinical encephalitis caused by EEE will die from the disease and of those who recover, many will suffer permanent brain damage with many of those requiring permanent institutional care.
In addition to humans, EEE virus can produce severe disease in: horses, some birds such as pheasants, quail, ostriches and emus, and even puppies. Because horses are outdoors and attract hordes of biting mosquitoes, they are at high risk of contracting EEE when the virus is present in mosquitoes. Human cases are usually preceded by those in horses and exceeded in numbers by horse cases which may be used as a surveillance tool.
EEE virus occurs in natural cycles involving birds and Culiseta melanura, in some swampy areas nearly every year during the warm months. Where the virus resides or how it survives in the winter is unknown. It may be introduced by migratory birds in the spring or it may remain dormant in some yet undiscovered part of its life cycle. With the onset of spring, the virus reappears in the birds (native bird species do not seem to be affected by the virus) and mosquitoes of the swamp. In this usual cycle of transmission, virus does not escape from these areas because the mosquito involved prefers to feed upon birds and does not usually bite humans or other mammals.
For reasons not fully understood, the virus may escape from enzootic foci in swamp areas in birds or bridge vectors such as Coquilletidia perturbans and Aedes sollicitans. These species feed on both birds and mammals and can transmit the virus to humans, horses, and other hosts. Other mosquito species such as Ae. vexans and Culex nigripalpus can also transmit EEE virus. When health officials maintain surveillance for EEE virus activity, this movement out of the swamp can be detected, and if the level of activity is sufficiently high, can recommend and undertake measures to reduce the risk to humans.
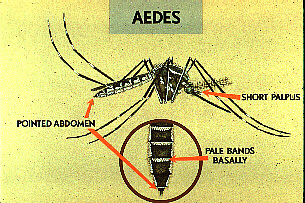 This mosquito belongs to the genus Aedes. Note the distinguishing features of the Aedes mosquitoes: pointed abdomen, short palpus, and pale bands at the base of each segment on the abdomen. |
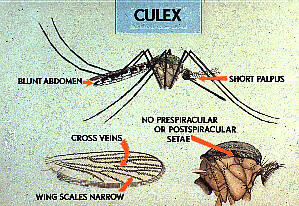 This mosquito belongs to the genus Culex. Note the distinguishing features of the Culex mosquitoes: cross veins on narrow wings, blunt abdomen, short palpus, and no prespiracular or postspiracular setae. |
Western Equine Encephalitis
The alphavirus western equine encephalitis (WEE) was first isolated in California in 1930 from the brain of a horse with encephalitis, and remains an important cause of encephalitis in horses and humans in North America, mainly in western parts of the USA and Canada. In the western United States, the enzootic cycle of WEE involves passerine birds, in which the infection is inapparent, and culicine mosquitoes, principally Cx. tarsalis, a species that is associated with irrigated agriculture and stream drainages. The virus has also been isolated from a variety of mammal species. Other important mosquito vector species include Aedes melanimon in California, Ae. dorsalis in Utah and New Mexico and Ae. campestris in New Mexico. WEE virus was isolated from field collected larvae of Ae. dorsalis, providing evidence that vertical transmission may play an important role in the maintenance cycle of an alphavirus.
Expansion of irrigated agriculture in the North Platte River Valley during the past several decades has created habitats and conditions favorable for increases in populations of granivorous birds such as the house sparrow, Passer domesticus, and mosquitoes such as Cx. tarsalis, Aedes dorsalis and Aedes melanimon. All of these species may play a role in WEE virus transmission in irrigated areas. In addition to Cx. tarsalis, Ae. dorsalis and Ae. melanimon, WEE virus also has been isolated occasionally from some other mosquito species present in the area. Two confirmed and several suspect cases of WEE were reported from Wyoming in 1994. In 1995, two strains of WEE virus were isolated from Culex tarsalis and neutralizing antibody to WEE virus was demonstrated in sera from pheasants and house sparrows. During 1997, 35 strains of WEE virus were isolated from mosquitoes collected in Scotts Bluff County, Nebraska.
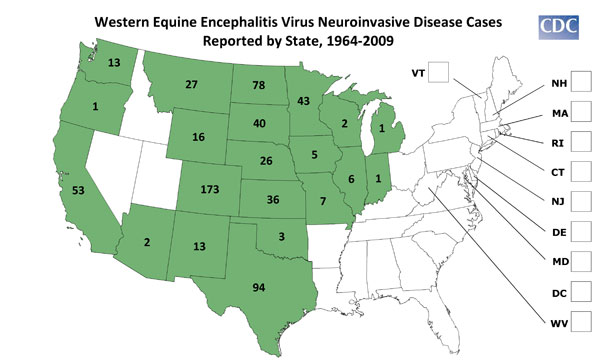
Human WEE cases are usually first seen in June or July. Most WEE infections are asymptomatic or present as mild, nonspecific illness. Patients with clinically apparent illness usually have a sudden onset with fever, headache, nausea, vomiting, anorexia and malaise, followed by altered mental status, weakness and signs of meningeal irritation. Children, especially those under 1 year old, are affected more severely than adults and may be left with permanent sequelae, which is seen in 5 to 30% of young patients. The mortality rate is about 3%.
St. Louis Encephalitis
In the United States, the leading cause of epidemic flaviviral encephalitis is St. Louis encephalitis (SLE) virus. SLE is the most common mosquito-transmitted human pathogen in the U.S. While periodic SLE epidemics have occurred only in the Midwest and southeast, SLE virus is distributed throughout the lower 48 states. Since 1964, there have been 4,437 confirmed cases of SLE with an average of 193 cases per year (range 4 - 1,967). However, less than 1% of SLE viral infections are clinically apparent and the vast majority of infections remain undiagnosed.
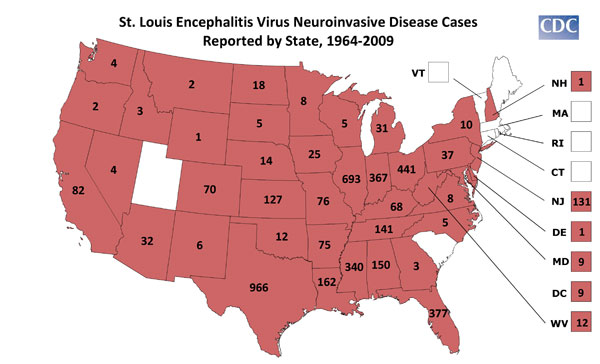
Illness ranges in severity from a simple febrile headache to meningoencephalitis, with an overall case-fatality ratio of 5-15 %. The disease is generally milder in children than in adults, but in those children who do have disease, there is a high rate of encephalitis. The elderly are at highest risk for severe disease and death. During the summer season, SLE virus is maintained in a mosquito-bird-mosquito cycle, with periodic amplification by peridomestic birds and Culex mosquitoes. In Florida, the principal vector is Cx. nigripalpus, in the Midwest, Cx. pipiens pipiens and Cx. p. quinquefasciatus and in the western United States, Cx. tarsalis and members of the Cx. pipiens complex.
Powassan Encephalitis
Powassan (POW) virus is a flavivirus and currently the only well documented tick-borne transmitted arbovirus occurring in the United States and Canada. Recently a Powassan-like virus was isolated from the deer tick, Ixodes scapularis. Its relationship to POW and its ability to cause human disease has not been fully elucidated. POW's range in the United States is primarily in the upper tier States.
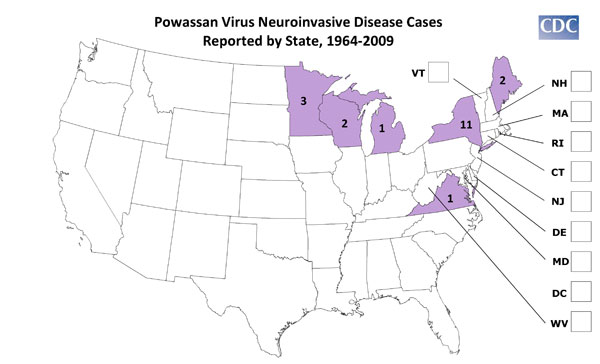
In addition to isolations from man, the virus has been recovered from ticks (Ixodes marxi, I. cookei and Dermacentor andersoni) and from the tissues of a skunk (Spiligale putorius). It is a rare cause of acute viral encephalitis. POW virus was first isolated from the brain of a 5-year-old child who died in Ontario in 1958. Patients who recover may have residual neurological problems.
Venezuelan Equine Encephalitis
Like EEE and WEE viruses, Venezuelan equine encephalitis (VEE) is an alphavirus and causes encephalitis in horses and humans and is an important veterinary and public health problem in Central and South America. Occasionally, large regional epizootics and epidemics can occur resulting in thousands of equine and human infections. Epizootic strains of VEE virus can infect and be transmitted by a large number of mosquito species. The natural reservoir host for the epizootic strains is not known. A large epizootic that began in South America in 1969 reached Texas in 1971. It was estimated that over 200,000 horses died in that outbreak, which was controlled by a massive equine vaccination program using an experimental live attenuated VEE vaccine. There were several thousand human infections. A more recent VEE epidemic occurred in the fall of 1995 in Venezuela and Colombia with an estimated 90,000 human infections. Infection of man with VEE virus is less severe than with EEE and WEE viruses, and fatalities are rare. Adults usually develop only an influenza-like illness, and overt encephalitis is usually confined to children. Effective VEE virus vaccines are available for equines.
Enzootic strains of VEE virus have a wide geographic distribution in the Americas. These viruses are maintained in cycles involving forest dwelling rodents and mosquito vectors, mainly Culex (Melanoconion) species. Occasional cases or small outbreaks of human disease are associated with there viruses, the most recent outbreaks were in Venezuela in 1992, Peru in 1994 and Mexico in 1995-96.
Other Arboviral Encephalitides
Many other arboviral encephalitides occur throughout the world. Most of these diseases are problems only for those individuals traveling to countries where the viruses are endemic.
Japanese Encephalitis
Japanese encephalitis (JE) virus is a flavivirus, related to SLE, and is widespread throughout Asia. Worldwide, it is the most important
cause of arboviral encephalitis with over 45,000 cases reported annually. In recent years, JE virus has expanded its geographic distribution
with outbreaks in the Pacific. Epidemics occur in late summer in temperate regions, but the infection is enzootic and occurs throughout the
year in many tropical areas of Asia. The virus is maintained in a cycle involving culicine mosquitoes and waterbirds. The virus is
transmitted to man by Culex mosquitoes, primarily Cx. tritaeniorhynchus, which breed in rice fields. Pigs are the main amplifying
hosts of JE virus in peridomestic environments.
 Distribution of Japanese Encephalitis in Asia, 1970-1998. |
 Culex mosquito laying eggs. |
Japanese encephalitis outbreaks are usually circumscribed and do not cover large areas. They usually do not last more than a couple of months, dying out after the majority of the pig amplifying hosts have become infected. Birds are the natural hosts for Japanese encephalitis. Epidemics occur when the virus is brought into the peridomestic environment by mosquito bridge vectors where there are pigs, which serve as amplification hosts, infecting more mosquitoes which then may infect humans. Countries which have had major epidemics in the past, but which have controlled the disease primarily by vaccination, include China, Korea, Japan, Taiwan and Thailand. Other countries that still have periodic epidemics include Viet Nam, Cambodia, Myanmar, India, Nepal, and Malaysia.
The incubation period of JE is 5 to 14 days. Onset of symptoms is usually sudden, with fever, headache and vomiting. Mild infections occur without apparent symptoms other than fever with headache. More severe infection is marked by quick onset, headache, high fever, neck stiffness, stupor, disorientation, coma, tremors, occasional convulsions (especially in infants) and spastic (but rarely flaccid) paralysis.
The illness resolves in 5 to 7 days if there is no CNS involvement. The mortality in most outbreaks is less than 10%, but is higher in children and can exceed 30%. Neurologic sequelae in patients who recover are reported in up to 30% of cases. A formalin-inactivated vaccine prepared in mice is used widely in Japan, China, India, Korea, Taiwan and Thailand. This vaccine is currently available for human use in the United States, for individuals who might be traveling to endemic countries.
Japanese encephalitis virus is NOT transmitted from person-to-person. For example, you cannot get the virus from touching or kissing a person who has the disease, or from a health care worker who has treated someone with the disease.
Tick-Borne Encephalitis
There are many different types of ticks in the United States, some of which are capable of transmitting infections. The risk of developing these infections depends upon the geographic location, season of the year, type of tick, and for Lyme disease only, how long the tick was attached to the skin.
While many people are concerned after being bitten by a tick, the risk of acquiring a tick-borne infection is quite low, even if the tick has been attached, fed, and is actually carrying an infectious agent. Ticks transmit infection only after they have attached and then taken a blood meal from their new host. A tick that has not attached (and therefore has not yet become engorged from its blood meal) has not passed any infection.
Tick-borne encephalitis (TBE) is caused by two closely related flaviviruses which are distinct biologically. The eastern subtype causes Russian spring-summer encephalitis (RSSE) and is transmitted by Ixodes persulcatus, whereas the western subtype is transmitted by Ixodes ricinus and causes Central European encephalitis (CEE). The name CEE is somewhat misleading, since the condition can occur throughout much of Europe. Of the two subtypes, RSSE is the more severe infection, having a mortality of up to 25% in some outbreaks, whereas mortality in CEE seldom exceeds 5%.
The incubation period is 7 to 14 days. Infection usually presents as a mild, influenza-type illness or as benign, aseptic meningitis, but may result in fatal meningoencephalitis. Fever is often biphasic, and there may be severe headache and neck rigidity, with transient paralysis of the limbs, shoulders or less commonly the respiratory musculature. A few patients are left with residual paralysis. Although the great majority of TBE infections follow exposure to ticks, infection has occurred through the ingestion of infected cows' or goats' milk. An inactivated TBE vaccine is currently available in Europe and Russia.
tick bite, note the pepperoni look
How to Remove a Tick
The proper way to remove a tick is to use a set of fine tweezers and grip the tick as close to the skin as is possible. Do not use a smoldering match or cigarette, nail polish, petroleum jelly (eg, Vaseline), liquid soap, or kerosene because they may irritate the tick and cause it to behave like a syringe, injecting bodily fluids into the wound.
The proper technique for tick removal includes the following:
- Use fine tweezers to grasp the tick as close to the skin surface as possible.
- Pull backwards gently but firmly, using an even, steady pressure. Do not jerk or twist.
- Do not squeeze, crush, or puncture the body of the tick, since its bodily fluids may contain infection-causing organisms.
- After removing the tick, wash the skin and hands thoroughly with soap and water.
- If any mouth parts of the tick remain in the skin, these should be left alone; they will be expelled on their own. Attempts to remove these parts may result in significant skin trauma.
West Nile Encephalitis
WNV is a flavivirus belonging taxonomically to the Japanese encephalitis serocomplex that includes the closely related St. Louis encephalitis (SLE) virus, Kunjin and Murray Valley encephalitis viruses, as well as others. WNV was first isolated in the West Nile Province of Uganda in 1937. The first recorded epidemics occurred in Israel during 1951-1954 and in 1957. Epidemics have been reported in Europe in the Rhone delta of France in 1962 and in Romania in 1996. The largest recorded epidemic occurred in South Africa in 1974.
Mapping Epidemics: A Historical Atlas of Disease (Reference)
An outbreak of arboviral encephalitis in New York City and neighboring counties in New York state in late August and September 1999, was initially attributed to St. Louis encephalitis virus based on positive serologic findings in cerebrospinal fluid (CSF) and serum samples using a virus-specific IgM-capture enzyme-linked immunosorbent assay (ELISA). The outbreak has been subsequently confirmed as caused by West Nile virus based on the identification of virus in human, avian, and mosquito samples. A recent outbreak WN encephalitis occurred in Bucharest, Romania in 1996.
The virus that caused the New York area outbreak has been definitively identified as a strain of WNV. The genomic sequences identified to date from human brain, virus isolates from zoo birds, dead crows, and mosquito pools are identical. SLE and West Nile viruses are antigenically related, and cross reactions are observed in most serologic tests. The isolation of viruses and genomic sequences from birds, mosquitoes, and human brain tissue permitted the discovery of West Nile virus in North America and prompted more specific testing. The limitations of serologic assays emphasize the importance of isolating the virus from entomologic, clinical, or veterinary material.
Although it is not known when and how West Nile virus was introduced into North America, international travel of infected persons to New York or transport by imported infected birds may have played a role. WNV can infect a wide range of vertebrates; in humans it usually produces either asymptomatic infection or mild febrile disease, but can cause severe and fatal infection in a small percentage of patients. Within its normal geographic distribution of Africa, the Middle East, western Asia, and Europe, WNV has not been documented to cause epizootics in birds; crows and other birds with antibodies to WNV are common, suggesting that asymptomatic or mild infection usually occurs among birds in those regions. Similarly, substantial bird virulence of SLE virus has not been reported. Therefore, an epizootic producing high mortality in crows and other bird species is unusual for either WNV or SLE virus. For both viruses, migratory birds may play an important role in the natural transmission cycles and spread. Like SLE virus, WNV is transmitted principally by Culex species mosquitoes, but also can be transmitted by Aedes, Anopheles, and other species. The predominance of urban Culex pipiens mosquitoes trapped during this outbreak suggests an important role for this species. Enhanced surveillance for early detection of virus activity in birds and mosquitoes will be crucial to guide control measures.
 This mosquito belongs to the genus Aedes. Note the distinguishing features of the Aedes mosquitoes: pointed abdomen, short palpus, and pale bands at the base of each segment on the abdomen. |
 This mosquito belongs to the genus Culex. Note the distinguishing features of the Culex mosquitoes: cross veins on narrow wings, blunt abdomen, short palpus, and no prespiracular or postspiracular setae. |
 This is a female Anopheles mosquito feeding on a person. Only the female mosquito feeds on blood. Notice how the body of this mosquito is held at an angle to the skin. |
Murray Valley Encephalitis
Murray Valley encephalitis (MVE) is endemic in New Guinea and in parts of Australia; and is related to SLE, WN and JE viruses. Inapparent infections are common, and the small number of fatalities have mostly been in children.